(debug: legal) The Pharma Legal Handbook: Saudi Arabia
The Pharma Legal Handbook: Saudi Arabia answers essential questions about the legal and regulatory environment for pharmaceuticals in Saudi Arabia. It is a must-have for any company operating in the country or looking to enter the market.
Prepared in association with STA, one of Saudi Arabia’s leading law firms, it should answer any questions linked to regulation, pricing, clinical and preclinical trials, marketing, manufacturing, trademarks and patents.
August 2021
1. Regulatory, Pricing and Reimbursement Overview: Saudi Arabia
(debug: permalink)1. What are the regulatory authorities with jurisdiction over drugs, biologicals, and medical devices in your country?
The Saudi Food & Drug Authority (SFDA) is the government agency that regulates drugs and medical devices in Saudi Arabia. It is also in charge of biological and chemical substances in Saudi Arabia.
2. What is the regulatory framework for the authorization, pricing, and reimbursement of drugs, biologicals, and medical devices?
Authorization:
The Saudi Food and Drug Authority (SFDA), the Drug Track and Trace System (RDS) track all human registered drugs that are manufactured in Saudi Arabia or imported to the country. RDS is a standardized identification system that tracks drugs from the manufacturer to the patient. It adopts GS1 standards and applies to all pharmaceutical products on the Saudi market, including over-the-counter (OTC) medicines. According to GS1, the Saudi Food and Drug Authority (SFDA) is working on similar requirements for medical devices. Saudi regulations stipulate that all drugs must be marked with a GS1 Data Matrix barcode that contains, at minimum, the GS1 Global Trade Identification Number (GTIN), the expiry date, and the batch/lot number. This information must also be printed on labels. All transactions for drug packages must be reported to a national Drug Track & Trace System (DTTS), and all manufacturers licensed by the SFDA must acquire a Global Location Number (GLN).
Pricing:
According to the new SFDA pricing guidelines, pharmaceutical products are to be priced taking into account:
- Therapeutic Value Add for the product (Value Add);
- Price of alternative products registered in KSA (Comparators);
- Ex-factory Price of the Manufacturer and Ex-factory to the countries where the product is marketed (Ex-f in its local currency);
- Wholesaler price in the COO (WSP in its local currency);
- Price to Public in the COO and the countries where the product is marketed (PP);
- Proposed Price to KSA (Cost, Insurance & Freight price i.e. CIF price in COO currency); CIF price to all countries where the product is marketed according to the official price certificate template. The price certificate validity has to be 6 months from the date of issuance;
- The price of the product is in the adopted price references.
Reimbursement:
The Ministry of Health (MOH) is the major government agency entrusted with the provision of preventive, curative and rehabilitative healthcare for the Kingdom’s population. The Ministry provides primary healthcare (PHC) services through a network of healthcare centres throughout the Kingdom. It also utilizes a referral system that provides curative care for all members of society from the level of general practitioners at health centres to advanced technology specialist/curative services through a broad base of general and specialist hospitals. The MOH also undertakes the overall supervision and follow-up of healthcare-related activities carried out by the private sector. Therefore, the MOH can be viewed as a national health service (NHS) for the entire population.
3. What are the steps to obtaining authorization to develop, test, and market a product?
Before being sold in the Kingdom of Saudi Arabia (KSA), medical devices must receive marketing authorization from the Saudi Food and Drug Authority (SFDA) under the Medical Devices Interim Regulation (MDIR), Decree No. 1-8-1429/2008.
Step 1
Appoint a Saudi Authorized Representative (AR) to manage your device registration in the KSA. Your representative must be licensed with the SFDA. Further, the SFDA must authorize the contract between you and your AR.
Step 2
Your Saudi AR must present the authenticated AR contract to the SFDA for review and obtain a license to represent you in the KSA. The AR contract and license may be valid for 1-10 years; however, the AR license cannot be valid longer than the contract.
Step 3
Prepare the Medical Device Marketing Authorization (MDMA) application and submit it through your AR. The application includes device labelling, IFU, promotional materials, proof of regulatory approval in your reference market and quality system certification (if applicable). Labelling, promotional materials, and IFU must be in English and Arabic; English only is acceptable for professional-use devices.
Step 4
The SFDA reviews the MDMA application for completeness. Then a third-party Conformity Assessment Body (CAB) performs a detailed technical review upon payment of the application fee.
Step 5
The SFDA makes the final decision based on recommendations of the CAB. Once the device is approved, the SFDA issues an MDMA certificate that you may provide to your distributor/importer for market entry.
Step 6
Device registrations are valid for three years, or the remaining validity is in the reference country you have chosen (if less than three years).
4. What are the approximate fees for each authorization?
The SFDA will charge fees for Regulatory Services of Pharmaceutical Products, according to the following:
- Issuing a Certificate of Pharmaceutical Product (CPP) or a Free Sale Certificate – 200 Saudi riyal
- Issuing a certificate for Good Manufacturing Practice (GMP) – 500 Saudi riyal
- Issuing a price list of company products – 500 Saudi riyal
- Evaluating product advertisement application – 14,000 Saudi riyal
- Inspecting a pharmaceutical consulting centre to issue a license – 1,000 Saudi riyal
- Inspecting a scientific office to issue a license – 5,000 Saudi riyal
- Pre-registration price estimation – 20,000 Saudi riyal.
5. For how long are marketing authorizations/registrations valid? How are marketing authorizations/registrations renewed?
Once the device is approved, the SFDA issues an MDMA certificate that you may provide to your distributor/importer for market entry. Device registrations are valid for three years, or the remaining validity is in the reference country you have chosen (if less than three years).
6. How does the authorization process differ between brandname products and generic products? Are there differences for local manufacturers versus foreign-owned manufacturers?
Marketing authorization for generic products is subject to the same legal process as brand-name products. Market authorizations from foreign jurisdictions are not recognized in Saudi Arabia, as all products are independently evaluated by the Saudi Food & Drug Authority (SFDA). However, importers or their agents must supply and sell pharmaceutical products from and to entities that have the applicable authorisation to deal in pharmaceutical products.
7. How are combination products (drug + drug, drug + biologic, drug + device, biologic + device, drug + biologic + device) regulated?
A product consists of two or more items that are subject to different SFDA’s jurisdictions in terms of regulatory path, marketing and/or manufacturing. It includes:
- Integrated combination product:
A product consists of two or more regulated components that are combined/ integrated as a single product. - Non-integrated combination product:
A product consists of two or more separate items that are contained in the same package. [Co-packaged combination product].
Any regulated product packaged separately where the labelling information refers to be used with another specific regulated product where both are required to achieve the intended purpose of use. [Cross-labeled combination product].
8. How is compliance with regulations monitored and evaluated? Is the regulatory regime comparable with the U.S. Food and Drug Administration or the European Medicines Agency expectations and requirements?
For medical devices, the SFDA is fully authorised to ensure compliance with the advertisement provisions, failing which the SFDA may withdraw or restrict the medical devices including taking any of the following actions:
- Suspending the licence.
- Terminating the licence.
- Recalling the product from the market.
- Withdrawing the marketing authorisation.
Yes, the regulatory regime is comparable with the US Food and Drug Administration and European Medicines Agency. Under the Registration of Products According to Verification and Abridged Process, if a drug is already licensed, SFDA will only accept it if the drug is approved by the US Food and Drug Administration (FDA) and/or the European Medicines Agency (EMA). There are two processes:
- Verification registration: a process where a product has been approved and marketed by both the FDA and EMA. The timeline is within 30 working days.
- Abridged registration: a process where a product has been approved and marketed by either the FDA or EMA. The timeline is within 60 working days.
9. What is the potential range of penalties for noncompliance?
Article 37 of the Regulations provides that the SFDA may impose the following penalties in respect of violations of the Regulations:
- Warning;
- A fine not exceeding SAR 100,000/-;
- Closure of the medical establishment for up to 60 days; and/or
- Cancelling the licence of the establishment.
10. Is there a national healthcare system? If so, how is it administered and funded?
“Health Care” in Saudi Arabia is a national health care system in which the government provides free health care services through several government agencies. There is also a growing role and increased participation from the private sector in the provision of health care services. The Ministry of Health is the major government agency entrusted with the provision of preventive, curative and rehabilitative health care for the Kingdom’s population.
11. How does the government (or public) healthcare system function with private sector healthcare?
Healthcare in Saudi Arabia is currently provided free of charge to all Saudi citizens and expatriates working in the public sector, primarily through the Ministry of Health and augmented by other governmental health facilities. The government requires that expatriates working in the private sector have some level of healthcare coverage paid by their employers. Healthcare has been seen as a “right”. Healthcare in Saudi Arabia has been funded primarily by the public (75%) or out-of-pocket expenditures (about 25%). What has been distinctive has been the low level of private insurance involved in the provision of healthcare. Almost all of the private expenditures have been out-of-pocket payments for services in private hospitals and clinics. Governmental funding is allocated through annual budgets to individual ministries and programs.
Health services in Saudi Arabia are provided through three main sectors: the MOH network of hospitals and primary healthcare centres that are distributed throughout the country, other governmental institutions, and the private sector. The MOH is the largest provider of healthcare services in the Kingdom, providing over 62% of inpatient care. Although the MOH is charged with the healthcare of the entire population, other governmental and private facilities are also important healthcare providers and provide over 20% and 17% of the inpatient facilities health services, respectively.
12. Are prices of drugs and devices regulated and, if so, how?
Saudi Arabia’s pharmaceutical market was valued at USD 8.3bn in 2019. Forecasts show by that by 2024 the market will clock USD 10.89 Billion with a CAGR of 5.4% Sales of patented drug consumption are supported by the population’s wealth and the preference for branded drugs among both consumers and prescribers, with patented drug spending accounting for 54.4% of the country’s drug market. It is the largest regional pharmaceutical market in overall value, but by drug per capita expenditure with USD242, it ranks seventh in the region. Saudi hospitals are among the best in the Middle East, while specialized tertiary facilities bear comparison with facilities in Western Europe. Large-scale plans for infrastructure developments exist, including new hospitals and health centres. Saudi Arabia’s epidemiological profile represents that of a developed country, with non-communicable diseases accounting for 84% of deaths, according to the WHO. Cardiovascular diseases (49%), ischemic heart disease (24%), and strokes (16%) are the three most prevalent chronic diseases by death.
13. How are the drugs and devices used by patients paid for? What roles do public and private payers play?
The Saudi health care sector is the largest in the Near East. Saudi Arabia’s health and social affairs budget for 2019 outlines an 8% increase to $46 billion as compared to $42.4 billion in 2018. This equates to about 15% of total government spending, budgeted at $260 million. Healthcare is the third-largest beneficiary behind education and the military.
Saudi Arabia’s healthcare landscape will continue to evolve as the government pursues reforms in the sector. Boosting health privatization initiatives will be a central part of Saudi’s economic diversification efforts, intended to reduce its dependence on the public sector. The healthcare sector contributed 4.7% to Saudi’s Gross Domestic Product (GDP) in 2018. The sector is projected to grow 13.7% by 2025. Recently, the government decided to privatize all its public hospitals and introduce a comprehensive insurance system for Saudi citizens as well as Public-Private Partnership (PPP) programs
The Kingdom of Saudi Arabia seeks to expand the private sector’s role in providing healthcare services under its ambitious Vision 2030 and its National Transformation Program. Saudi Arabia’s deteriorating environment for intellectual property protections in pharmaceuticals, however, dampens the positive signals sent to entrepreneurs, investors, and innovators in the private sector. In recent years, the Saudi Arabia Food and Drug Authority (SFDA), which the Minister of Health oversees, has authorized domestic companies to produce generic versions of pharmaceutical products that are under patent protection either in Saudi Arabia or the Gulf Cooperation Council (GCC).
14. Who dispenses drugs and devices to patients and how are those dispensers compensated?
A public health service provides coverage for medicines that are on the Saudi National Formulary for inpatients and outpatients. Under Saudi law, there is no limit for medical benefits, in other words, citizens have the right to access all kinds of medicines. Private health insurance schemes provide medicines coverage. They are required to provide at least partial coverage for medicines that are on the Saudi National Formulary.
15. What are the professional and legal responsibilities of those who dispense drugs and devices? What role do they play in providing patient care, information, and safety?
Co-payments or fee requirements for consultations are not levied at the point of delivery. Furthermore, there are no co-payments or fee requirements imposed for medicines. In Saudi Arabia, there are legal or regulatory provisions affecting the pricing of medicines. These provisions are aimed at the level of manufacturers, wholesalers and retailers. They affect: Innovated Patented Products, Generic Products, Under License Locally Manufactured Products, Products with Different Package Sizes or Strengths, Products that have Specific Advantages, Fixed Combination Drug Product. The government runs an active national medicines price monitoring system for retail prices. Regulations exist mandating that retail medicine price information should be publicly accessible. The retail price is published on the SFDA website, or the price can be printed on the outer packaging of the medicine.
Also from this Legal Handbook
2. Preclinical and Clinical Trial Requirements: Saudi Arabia
(debug: permalink)1. Are clinical trials required to be conducted locally as a condition (stated or implicit) for marketing approval?
It is mandatory to inform SFDA immediately about any Suspected Unexpected Serious Adverse Reactions (SUSAR) as soon as possible, no later than 15 days. If the SUSAR is fatal or life-threatening, SFDA must be informed as soon as possible, no later than seven days following (ICH-E2A) guideline.
2. How are clinical trials funded?
A total of 80 sponsors funded the clinical studies in Saudi Arabia. The majority of the clinical trials are funded by multinational pharmaceutical companies. Oncology (13.81%) and diabetes (11.71%) were the most common therapeutic areas and constituted the largest proportion of the overall studies.
3. What are the requirements for preclinical and clinical trial protocols? Who must approve the protocols?
“The Saudi Food and Drug Authority” have created a state of the artstate-of-the-art regulatory framework that supports quick approval of clinical trials. Saudi Clinical Trials Registry (SCTR) is an online record system for the registration of clinical trials being conducted in Saudi Arabia. The requirements for the clinical and pre-clinical trial are:
- Headed Letter to SFDA
- SCTR registration
- Bank Transfer Payment (for non-governmental)
- Confidentiality Agreement
- Trial Protocol
- Informed Consent Form
- IRB/EC Approval
- Investigator Brochure (include preclinical data)
- Financial Disclosure of Principal Investigator
- GMP Certificate
- Certificate of Analysis of Study Drug
- Labelling of Study Drug
- Clinical Trial Agreement
- CVs of Principal Investigator & Coordinator
- Case Report Form
- Subjects Insurance
- Delegation/Authorization Letter (for CRO)
4. What are the requirements for consent by participants in clinical trials?
Informed consent is a process by which the participant voluntarily confirms his/her willingness to participate in a particular trial, after having been informed of all aspects of the trial that are relevant to the participant’s decision to participate in a clinical trial. Informed consent has the potential as a risk management activity to ensure that patients have been provided with adequate information concerning the study as well as the risks of the medicine and appropriate Clinical Trial Authorization Process.
5. May participants in clinical trials be compensated?
Participants in clinical trials did not receive any compensation.
6. How are participants in clinical trials protected and indemnified against any harm that arises as a result of participation in the trial?
N/A
Also from this Legal Handbook
3. Marketing, Manufacturing, Packaging & Labeling Advertising: Saudi Arabia
(debug: permalink)1. What is the authorization process for the marketing of new drugs, biologics, medical devices, over-the-counter medications, and other medicinal products?
The MAA of the pharmaceutical product will be subjected to the followings processes:
- Submit the application form and pay the fees.
- Upload the product file; The components of the file shall follow the requirements and guidelines published on the SFDA website.
- Validation The product file will be validated on a technical and business basis to ensure the applicant fulfils the requirement.
- Testing 1. The registration request will be forwarded to the SFDA Central Laboratories. 2. If more information or clarification is required, an electronic inquiry will be posted through the SDR system.
- Pricing: The Pricing Department will review the product’s price according to the “SFDA’s pricing rule.
2. What is the authorization process for the marketing of generic versions of these products?
Saudi Arabia’s generic drug market is supported by the government’s encouragement of generic substitution as a means to control costs thereby acting as a significant growth driver of this trend.
3. What are the typical fees for marketing approval?
Please see Question 4 in the Regulatory, reimbursement and pricing overview.
4. What is the period of authorization and the renewal process?
An applicant shall submit a renewal request every five years. It is possible to request renewal within six months of the certificate expiry.
5. What are the requirements, if any, for post-approval pharmacovigilance?
The role of the Pharmacovigilance advisory committee is to provide advice on the safety of medicinal products and the investigation of adverse reactions, to enable effective risk identification, assessment and management, in the pre-and post-authorization phase leading to recommendations on the action at the request of the SFDA for products available in Saudi Arabia.
Detailed Description of the Pharmacovigilance System to Be Included in the Marketing Authorisation Application Proof of the Services of a QPPV and the Necessary Means to Notify Adverse Reactions, to be Included in the Marketing Authorisation Application. The MAH should ensure that they have an appropriate system of pharmacovigilance in place to assure responsibility for their products on the market and to ensure that appropriate action can be taken, when necessary.
6. Are foreign marketing authorizations recognized?
No, foreign marketing authorizations are not recognized.
7. Is parallel import of medicines or devices allowed?
It is illegal to import drugs or medical materials that are ban in Saudi Arabia or internationally. It is illegal to import drugs in Article (4) of the Drugs and Narcotics Control Law (found at the website of the Saudi Food and Drug Authority.
Request to import prescription drugs must be filed with the branch of the Saudi Food and Drug Authority at the port of entry to which the drugs will arrive.
8. What are the restrictions on marketing practices such as gifts, sponsorships, consultancy agreements, travel and entertainment, or other incentives for healthcare organizations and individual medical practitioners?
- It is not permissible for the healthcare practitioner, whether in the public or private sector to accept or give bribes, including accepting gifts that are linked to the number of prescriptions that he/she prescribes or the number of equipment he/she advises his/her patients to have; that is then reflected as a benefit to the corporate interest.
- It is not permissible for the healthcare practitioner to accept gifts, loans, equipment, instruments, or cash paid directly to him/her personally from the commercial companies for whatever justification.
- The healthcare practitioner can accept inexpensive gifts like pens and the like, such as books, or medical journals if they were presented to him/her in a non-personal way, on the condition that it is not linked to any advertisement for a specific product.
- The healthcare companies or institutions for which the healthcare practitioner works can accept educational grants and financial support to attend training courses, conferences, or other activities that these companies or institutions choose for the healthcare practitioners according to the public interests.
- On a personal basis, the healthcare practitioner is not allowed to accept subsidies to compensate his/her travel expenses, accommodation, or meals when attending symposia and conferences, or compensation on the time he/she spent to attend the training. He/she can accept the meals offered to all participants.
9. How is the manufacturing of medicines and devices regulated and by which agencies?
The Pharmaceuticals Law sets out the requirements for the manufacture of medicines. Without a license ordained by the Ministry of Health, no one is allowed to open such a factory.
10. Are local manufacturing requirements compatible with Good Manufacturing Practices (GMPs) as defined by the US Food & Drug Administration (US FDA) and/or the European Medicines Agency (EMA)?
Good manufacturing practice (GMP) is a system for ensuring that products are consistently produced and controlled according to quality standards. It is designed to minimize the risks involved in any pharmaceutical production that cannot be eliminated through testing the final product in Saudi Arabia. There must be systems to provide documented proof that correct procedures are consistently followed at each step in the manufacturing process – every time a product is made in Saudi Arabia. WHO has established detailed guidelines for good manufacturing practices. Many countries have formulated their requirements for GMP based on WHO GMP.
11. What is the inspection regime for manufacturing facilities?
The Ministry of Health and Prevention offers inspection to ensure compliance with GMPs as a business service – an applicant may file a request for an inspection of a pharmaceutical manufacturing establishment. This service is part of a quality assurance system that ensures the quality of pharmaceutical and medical products by controlling all activities associated with Good Storage Practices and Good Clinical Practices.
12. Are manufacturing facilities open for inspection by foreign inspectors or third-party inspectors as authorized by the FDA/EMA?
Yes, manufacturing facilities are open for inspection by foreign or third-party inspectors.
13. What are the requirements for storage, packaging, and handling of medicines and devices and their constituent components?
The Saudi Food and Drug Authority (SFDA), the requirements for storage, packaging, handling and transportation of medicines and devices, including the following:
- The instruments for measuring the temperature and humidity of storage areas (including vehicles used for transportation) are now required to be electronic, to allow both monitoring and the adjustment of values according to instructions provided by the medical device manufacturer.
- Temperature mapping instruments must be capable of connecting to the electronic systems used by the SFDA.
- The SFDA has provided a list of eligible facilities that provide temperature and humidity management and tracking services.
- For most devices, Medical Device Marketing Authorization (MDMA) approval from the SFDA is required before placing a product on the market.
14. What information must be included in medicine and device labeling?
Medical devices are subject to specific labelling guidelines. The device or the packaging, as appropriate, must be labelled with the name of the device, name and address of the manufacturer, special storage conditions, warnings and contra-indications, and batch code or lot number, along with many other requirements specific to the type of device. Complete labelling guidelines are available from the Medical Device department at the Saudi Food and Drug Authority.
15. What additional information may be included in labeling and packaging?
Concerning the medicines, the Code provides that the labelling and packaging of pharmaceutical products should include, among other things, the following:
- Trade name and a generic name.
- Name and address of the company/agent responsible for marketing.
- Usage, dosage and method of use.
16. What items may not be included in labeling and packaging?
Goods bearing immodest or nude pictures or bearing a cross and pictures not consistent with Islamic morals are prohibited. There should be no Koranic or Islamic sayings written on packages, other than straight translations of product contents, when necessary. If human figures are shown on packages, they should be in good taste. Photographs or illustrations of pigs or wrappings simulating pigskin should be avoided. Permission must be obtained for goods to bear the Saudi Arabian emblem, which consists of crossed swords and a palm.
17. What are the restrictions and requirements for the marketing and advertising of medicines and devices?
MEDICINES:
As per the Rules, the following are some of the restrictions and controls applicable to the advertisement of non-prescriptive pharmaceutical products to the general public:
- Only non-prescriptive products can be advertised.
- The product should be registered with the SFDA.
- Advertisements should only be made during the validity of the respective approval. The validity of approval for advertising products is one year.
- Advertisements should comply with the principles of Sharia and should not violate public decency.
- Advertisements should not provide incorrect or misleading information, or information that is unclear and that is susceptible to inappropriate interpretation.
DEVICES:
Article 41 of the Interim Regulations provides for restrictions applicable to the advertising of medical devices as follows:
- The advertising of a medical device for which the SFDA has not issued a marketing authorisation is prohibited.
- All advertisement material must be approved by the SFDA.
- The advertising material must not mislead the user regarding the performance of the medical device as specified by the manufacturer.
- The advertising to the general public, including on the internet, must avoid misleading laypersons.
- Any advertising to persons qualified to use medical devices must include the relevant information compatible with their specific needs.
- Medical sales representatives must have sufficient knowledge to be able to provide appropriate information about the medical devices they promote.
18. Where can medicines and devices be sold or delivered? Can medicines and devices be sold or delivered via post?
Prescription drugs
Prescription-only medicine (POM) refers to a medicinal product that is subject to the Saudi Food and Drug Authority’s (SFDA) authorisation and is only available to patients in Saudi Arabia based on a licensed physician’s prescription. Places, where patients/consumers can purchase or gain access to medicinal products (pharmacies and hospitals), are authorised to distribute POMs to consumers.
Over-the-counter (OTC) products
An OTC product is a medicinal product subject to SFDA authority that can be obtained by patients in Saudi Arabia without a physician’s prescription. OTC products are distributed in pharmacies. Further, some pharmaceutical products are permitted by the SFDA to be sold in food retail stores in cities (including medium-sized grocery stores, large grocery stores and supermarkets) and highways that have the proper storage conditions and requirements for pharmaceutical products 24/7 and are allowed to sell pharmaceutical products individually.
Certain medicines such as prescription-only medicines, are only available to patients in Saudi Arabia based on a licensed physician’s prescription and therefore cannot be offered through distance selling or via post.
19. What are the restrictions and requirements for electronic marketing and advertising via email, by internet, social media, and other channels?
Pharmaceutical products can be advertised via social media or television. Pharmaceutical companies and health care providers must ensure that the information provided to consumers is accurate, balanced, true and conforms with the promotional standards (Saudi Code of Pharmaceutical Promotional Practices).
The Rules provide that online advertisements for non-prescriptive pharmaceutical products should be available on the webpage provided by the applicant, and should not redirect visitors to other websites. It is further provided that online advertisements should not include information about medical practitioners. Concerning the medical devices, Interim Regulations provide that the advertising and marketing material must avoid misleading laypersons, when advertising to the general public, including advertisements on the internet.
20. May medicines and devices be advertised or sold directly to consumers?
Yes, it is possible to sell or advertise medicines and devices directly to consumers. Direct-to-Consumer Advertising (DTCA) of pharmaceuticals, defined as “any presentation or promotion of the information of the prescribed medications in media to the general population” has gained increasing attention over the past two decades, enhancing consumer access to prescription drug information.
21. How is compliance monitored?
Please refer to Question 8 of Regulatory, pricing and reimbursement overview.
22. What are the potential penalties for noncompliance?
Please refer to Question 9 of Regulatory, pricing and reimbursement overview.
Also from this Legal Handbook
4. Traditional Medicines and OTC Products: Saudi Arabia
(debug: permalink)1. What are the regulatory requirements for traditional, herbal, complementary, or alternative medicines and devices?
This presentation comprised a briefing on the current situation of the practice of traditional medicine (TRM) and complementary and alternative medicine (CAM), the availability of two kinds of herbal products, from local and imported crude medicinal plants, and the history of traditional medicine in Saudi Arabian culture.
2. Can these traditional, herbal, complementary, or alternative products be advertised directly to the public?
Yes, but the national authorities responsible for the regulation of herbal medicinal, traditional, complementary products and practices should authorize every advertisement before it reaches the public.
The regulatory authority should issue an advertising permit after a satisfactory evaluation of the contents of the advert to ensure that the public gets the correct information about the product, devoid of ambiguous or false claims. The print and electronic media should be notified to ensure that every advertiser of herbal medicinal products obtains the advertising permit from the national authority before such advertisements are circulated.
3. What health, advertising, and marketing claims may be made for traditional, herbal, complementary, or alternative products?
Medical claims: Medical claims are here defined as those claims specified to treat, cure or prevent a disease or restore, correct or modify physiological functions. Frequently, products with medical claims have to be registered by the medical products agency before being allowed onto the market.
Health claims: Health claims could, for instance, include “any statement, suggestion, or implication in labelling or advertising that a product carries a specific health benefit, but not nutritional claims nor medical claims. The term “health claims” further includes claims which refer to nutrient function and recommended dietary practice.
Nutrient content claims: Nutrient content claims, for instance, indicate that a certain product is particularly rich or low in a nutritional component such as fibre or fat.
4. What are the regulatory requirements for over-the-counter (non-prescription) medications?
Pharmacies in Saudi Arabia are easily accessible to the public. Antibiotics, as well as many other non-prescription medications, are available OTC. Another study examined the behaviour of parents with regards to OTC medication and found that, out of 750 parents from different cities across Saudi Arabia, 80% self medicate their children. This correlates with the findings of the present study, which indicated that the majority of respondents (67.2%) practice self-medication with OTC drugs.
This is not surprising as many patients in Saudi Arabia can buy any OTC drug without any prescription. The current study revealed that the majority of patients use the OTC drugs without the advice of a health professional, as customers felt shy and reluctant, there was a lack of confidentiality, the pharmacist was felt to be impatient, there was a queue, or the customer did not trust the pharmacist.
5. Are there any limitations on locations or channels through which OTC products may be sold?
Please refer to Question 4 above.
6. What health, advertising, and marketing claims may be made for OTC products?
The medical advertisements must contain the generic name, a statement of “Please purchase and use following the drug instructions or under the guidance of pharmacist”, its medical advertisement approval number and its manufacturing approval number. Meanwhile, a special label (OTC) for OTC drugs should be indicated in the advertisements for such non-prescription drugs.
7. Can OTC products be marketed or advertised directly to the public?
It is permitted to advertise OTC medication to the general public in Saudi Arabia. However, it is subject to the prior approval of the SFDA. The content of advertisements is examined by the SFDA and after obtaining approval, the SFDA approval number must be displayed in the advertising.
8. What is the mechanism by which a prescription-only product can be converted to an OTC product?
The SFDA has the authority to revise and publish the list of OTC drugs. Alternatively, the marketing authorization holders can apply to the SFDA for the conversion between prescription drugs and OTC drugs.
9. What are the requirements for the importation of either traditional medicines or OTC products?
The requirements for the importation of either traditional medicines or OTC products are generally the same as those for chemical drugs or prescription drugs.
Also from this Legal Handbook
5. Product Liability: Saudi Arabia
(debug: permalink)1. What types of liability are recognized in your jurisdiction?
Product liability is the area of law in which manufacturers, distributors, suppliers and retailers are held responsible for any injuries products cause. Generally, there are three liabilities: Civil liability, punitive liability and disciplinary liability recognised in Saudi Arabia.
2. How do these types of liabilities apply to the manufacturers of medicines and devices?
The Consumer Protection Association of Saudi Arabia aims at protecting civil liability by protecting the consumer’s interests, safeguarding and defending consumer rights; protecting him/her against all kinds of adulteration, counterfeit, fraud, deceit, falsification and exaggerated prices; and promoting consumer awareness and rationalized consumption.
Punitive liability is applicable when manufacturing, acquiring, displaying or selling products that do not conform to applicable standards specifications.
Disciplinary liability is when a manufacturer failed to meet professional standards, requirements, and ethics.
3. Does potential liability extend to the manufacturer only or could claims extend to corporate executives, employees, and representatives?
Potential liability may also extend to corporate executives, employees and representatives.
4. How can a liability claim be brought?
A manufacturer is held liable for faults in its products and can be required to repair or replace the product as applicable, as well as to compensate for the harm caused by using the relevant products. A liability claim can be brought by way of a regular lawsuit in civil proceedings before a court.
5. What defenses are available?
Product defence includes manufacturing products that should follow the guidelines of the Saudi Standards, Metrology and Quality Organisation.
Also from this Legal Handbook
6. Patents & Trademarks: Saudi Arabia
(debug: permalink)1. What are the basic requirements to obtain patent and trademark protection?
Trademarks, copyright works, patents, industrial designs and domain names are all capable of being protected in Saudi Arabia and there is an established legal framework that supports the registration and records of these valuable IP rights. Protection can take place through two primary means:
Firstly, through registration or records of the right through a government authority in Saudi Arabia.
Secondly, IP rights can, and should also be protected through contracts such as non-disclosure agreements, licensing agreements or development agreements.
2. What agencies or bodies regulate patents and trademarks?
The primary legislation governing trademarks in Saudi Arabia are:
- The Gulf Cooperation Council Trademark Law, which entered into force on 27 September 2016 by Royal Decree M/51 of 26.07.1435H and its implementing regulations;
- The Anti-Commercial Fraud Law, promulgated by Royal Decree M/19 of 23.04.1429H (29 April 2008) and its implementing regulations issued by Ministerial Resolution 155 of 06.01.1431H; and
- The Border Measures Regulations issued according to Ministerial Resolution;
- The GCC Patents of Inventions Regulation of 2001, which is an amendment of an earlier statute of 1992, was approved in Saudi Arabia by Royal Decree No M/28 of 2001. This permits the registration of patents with effect throughout the GCC country.
3. What products, substances, and processes can be protected by patents or trademarks and what types cannot be protected?
Under Saudi trademark law, a trademark can be anything that takes a distinctive shape, such as names, words, signatures, letters, symbols, numbers, titles, stamps, drawings, pictures, inscriptions, packaging, figurative elements, shapes or colours, groups of colours or combinations thereof; or any sign or group of signs used or intended to be used to distinguish the goods or services of one undertaking from those of others, or intended to identify a service, or used as a certification mark in respect of goods or services. An invention can be patented if it has a useful purpose, has patentable subject matter, is novel, and is non-obvious. The patent could cover a composition, production process, the machine, tool, new plant species, or an upgrade to an existing invention.
- Inventions that cannot be patented:
- Computer programs as such cannot be patented under Saudi law;
- Discoveries, scientific theories and mathematical methods;
- Aesthetic creations (such as literary, dramatic, or artistic works);
- Schemes or methods for performing a mental act;
- Games or business methods;
- Presentation of information;
- Plant varieties, animal species and biological methods of producing plants or animals, except for microorganisms, non-biological and microbiological processes.
Trademarks cannot be registered with goods and services that are forbidden in Saudi Arabia. For example, pork products and alcoholic beverages are banned in this country.
4. How can patents and trademarks be revoked?
PATENTS
Patents can be revoked on the following grounds.
- The invention lack novelty at the time of filing;
- The invention lacks inventive steps;
- The invention is not industrially applicable;
- The subject matter of the patent is hit by exclusionary subject matters (ie, the subject matter is not capable of being patented);
- Commercial exploitation of the invention violates Islamic principles.
- Commercial exploitation of the invention is harmful to life; to human, animal or plant health; or the environment.
TRADEMARKS
A trademark that is not used for consecutive five years can be cancelled by way of a non-use cancellation action before the Administrative Court of First Instance. The trademark owner can defend such action by providing reasonable justification for non-use, which may relate to reasons beyond its control, such as war, import sanctions or any other justifiable reason that can prove that it had no intention of stopping the use of the registered mark. A single invoice has been accepted as sufficient evidence to defeat a non-use cancellation action in Saudi Arabia.
A registered trademark can also be cancelled through a cancellation action before the Administrative Court of First Instance if the trademark was unlawfully registered. There is no definition of such unlawfulness in the law; however, prior registration and prior use can be strong grounds for a cancellation action.
5. Are foreign patents and trademarks recognized and, if so, under what circumstances?
Foreign patent and trademark registrations are recognised in Saudi Arabia as evidence of good faith ownership if combined with a claim of prior use in Saudi Arabia. Among others, foreign trademark registration is one form of evidence that helps to establish the global fame of a trademark in Saudi Arabia.
6. Are there any non-patent/trademark barriers to competition to protect medicines or devices?
No, there are no non-patent/trademark barriers to competition to protect medicines or devices.
7. Are there restrictions on the types of medicines or devices that can be granted patent and trademark protection?
There are no restrictions on the types of drugs or devices that can be protected through patents or trademarks other than those already described.
8. Must a patent or trademark license agreement with a foreign licensor be approved or accepted by any government or regulatory body?
No, it is not mandatory.
Also from this Legal Handbook
7. Regulatory Reforms: Saudi Arabia
(debug: permalink)The key facts about Regulatory Reforms in Saudi Arabia. Prepared in association with STA, an international law firm, this is an extract from The Pharma Legal Handbook: Saudi Arabia, available to purchase here for GBP 99.
1. Are there proposals for reform or significant change to the healthcare system?
The government is now ardently pursuing private sector development and has initiated privatization and marketization as a core strategy of reforms in its health system. The review has indicated that by mid-2019, the reform has contributed to an increase of 37.5% in the rate of PHC visits and 4.7% increase in patient satisfaction, enhanced coverage of rural communities (from 78% to 83%), and contributed to increasing the screening rate for prevalent chronic diseases.
PHC reform process in Saudi Arabia has demonstrated that positive change is achievable. This has been aided by building on previous accomplishments and the wealth of experience gained throughout the PHC journey in Saudi Arabia. However, despite improvement in the quality of services, continuous improvement is required to meet the rising expectations of the population.
2. When are they likely to come into force?
The Saudi Arabian Ministry of Health embarked on reforming its health sector as part of a wider agenda for transforming all government sectors as envisioned in Vision 2030 and the National Transformation Programme 2020. In line with Vision 2030, the reform agenda proposed under the National Transformation
Programme 2020 further underlines the importance of bridging the gap to accessing health services, emphasizing universal health coverage.
Also from this Legal Handbook
8. Cannabinoid Drugs, Medicinal Cannabis and Opioid Drugs: Saudi Arabia
(debug: permalink)Cannabinoid drugs, medicinal cannabis and opioid drugs in Saudi Arabia – a comprehensive legal overview.. Prepared in association with STA, an international law firm, this is an extract from The Pharma Legal Handbook: Saudi Arabia, available to purchase here for GBP 99.
Cannabinoid Drugs
1. Are Cannabinoid Drugs authorized in your country?
Cannabinoid Drugs are not authorized in Saudi Arabia.
2. What are the regulatory authorities with jurisdiction over Cannabinoid Drugs?
The concerned authorities are the Saudi FDA and the Saudi Customs Authority.
3. Is there a specific regulatory framework for the authorization, pricing, and reimbursement of Cannabinoid Drugs?
There is no regulation on cannabinoids.
4. Which are the cannabinoid drugs that have received market approval to date?
None.
5. Who can prescribe Cannabinoid Drugs?
Not legally permissible.
6. Is there a list of doctors authorized to prescribe Cannabinoid Drugs?
No.
7. What approvals or notifications are required to prescribe Cannabinoid Drugs?
Approvals are not given.
8. Which organizations are authorized to sell/distribute Cannabinoid Drugs available?
No authorized distributers or resellers within Saudi Arabia.
9. Is there a list of retailers/distributors authorized to sell Cannabinoid Drugs?
No, retailers and distributers are not authorized to sell cannabinoid drugs in Saudi Arabia.
10. Are there proposals for reform or significant change to the regulation of Cannabinoid Drugs?
Not as of present.
11. When are they likely to come into force?
Not known, Indefinite.
Medicinal Cannabis
12. Is Medicinal Cannabis authorized in the country?
No, it is not authorized in Saudi Arabia.
13. What are the regulatory authorities with jurisdiction over Medicinal Cannabis?
There are none owing to its illegality in Saudi Arabia.
14. What is the regulatory framework for the authorization, pricing, and reimbursement of Medicinal Cannabis?
The authorization, pricing, and reimbursement of Medicinal Cannabis is not applicable in Saudi Arabia, as it is considered to be illegal in all its forms.
15. How is the production and import of Medicinal Cannabis regulated and by which agencies/authorities?
There is no production regulation.
16. What approval or notifications are necessary to produce or import Medicinal Cannabis?
Not applicable.
17. What is the regulatory framework for the marketing and distribution of Medicinal Cannabis?
Not applicable.
18. How can patients obtain Medicinal Cannabis?
Not applicable.
19. Who can prescribe Medicinal Cannabis?
Not applicable.
20. Is there a list of doctors authorized to prescribe Medicinal Cannabis?
There isn’t a formulated list for the same.
21. What approvals or notifications are required to prescribe Medicinal Cannabis?
Not applicable.
22. Where is Medicinal Cannabis available?
Not applicable.
23. Is there a list of retailers authorized to sell Medicinal Cannabis?
Not applicable.
24. Are there proposals for reform or significant change to the regulation of Medicinal Cannabis?
Not applicable.
Opioid Drugs
25. Are Opioid Drugs authorized in your country?
Prescribed opioids may be permitted under the below mentioned:
Article (5): Clearance of Pharmaceutical Products for Personal Use
- It is illegal to import drugs or medical materials that are banned in Saudi Arabia or internationally.
- It is illegal to import drugs listed in Table 1 in Schedule D and Table 2 in Schedule A, as well as items listed in Article (4) of the Drugs and Narcotics Control Law (found at the website of the Saudi Food and Drug Authority.
- Request to import prescription drugs must be filed with the branch of the Saudi Food and Drug Authority at the port of entry to which the drugs will arrive.
- Drugs that are for personal use will be cleared for import into the Kingdom of Saudi Arabia provided the following conditions are met:
a. The prescription medications in question must be accompanied either:
+ Recent medical report (less than six months old) issued by the patient’s medical care provider and clearly stating the following:
- personal information of the patient.
- medical diagnosis;
- treatment plan;
- medical recommendations;
- generic name of the prescription drugs, dosage and dosage form;
or by
+ A doctor’s prescription (less than six months old) in the name of the patient with the following information:
- medical diagnosis;
- generic name of the prescription drug, dosage and dosage form;
- drug usage instructions and prescribed duration of use; and
- official seal of the health care provider.
b. The person importing the prescription medications will be held personally responsible for its lawful use and agrees to limit its usage to the intended patient only.
c. Copy of patient identification document.
5. The amount of allowable prescription medicine to be cleared for import shall for the duration of the visit or one month’s supply, whichever is shorter. The prescription medications must remain valid for use during the clearance period and satisfy the following conditions:
a. In case the quantity of the cleared prescription medications were used up while the patient is in the Kingdom, he/she should visit a physician in a licensed medical facility to verify his/her need to continue on the same drug.
b. Should the physician confirm such need, a medical file for the patient should be opened at the medical facility before a prescription can be issued. The prescribed drug must then be dispensed by a local pharmacy, if available. This same procedure must be followed every time the patient’s condition requires medication.
c. If the prescription medications or its medically acceptable replacement is not available in the local market, the medical facility prescribing the medication may request permission from the Saudi Food and Drug Authority to import the medication from a pharmaceutical distributor.
6. If the prescription medication is used through injection, the clearance process should be completed on behalf of the patient by and under the supervision of a local medical institution. The cleared prescription medications should then be registered in the record of the medical institution for personal use in accordance with its medication management policy.
7. Should the quantity of the cleared medication exceed the medical need of the patient, unused medication must be disposed of in a proper manner.
8. With the exception of the conditions provided for under subsections (a), (b) and (c) of Sections (5) and (6), all other conditions shall similarly apply to those patients who are travelling outside the Kingdom of Saudi Arabia.
9. If the prescription drug is in the possession of someone other than the patient (spouse, parents, children, or siblings) a copy of that person’s identification must be submitted with the clearance application. However, if that person is not a relative he/she must submit a document showing patient’s consent or authorization for him/her to handle the patient’s medications along with a copy of his/her identification.
26. What are the regulatory authorities with jurisdiction over Opioid Drugs?
The regulatory bodies under concern are the Saudi FDA, Ministry of Health and the Saudi Ministry of Customs.
27. Is there a specific regulatory framework for the authorization, pricing, and reimbursement of Opioid Drugs?
Yes, there is specific regulatory framework for the authorization, pricing, and reimbursement of Opioid Drugs.
28. Which are the Opioid drugs that have received market approval to date?
Controlled medications refer to a group of medications that besides being internationally recognized scheduled drugs, includes other psychoactive drugs as defined by the Saudi Ministry of Health regulations.
Consultants, assistants, dentists, and fellows may prescribe controlled medications, only if the use of such drugs falls under their area of specialization or patient management.
Inpatients
- Residents.may initiate new or discharge orders, but they must write the attending consultant’s name and ID next to their signatures. Consultants must countersign such orders within 24 hours.
- Residents may renew or alter prescriptions initially written by consultants, assistants, or fellows for adjustments in dose or frequency based on clinical status of the patient.
Outpatients
- A maximum 90-day supply of controlled medications may be dispensed at any one time. The total quantity to be dispensed must not exceed the medically recommended maximum daily dose for 90 days. For Emergency Room patients, a maximum of 30 individual units or liquid doses shall be dispensed.
- Physicians are not to prescribe narcotics or controlled medications for self or direct family use but have to utilize family health or routine clinic system for such medications. All such prescriptions must be noted in the individual medical charts.
- Emergency clinic prescriptions for new patients without KFSH number/addressograph must have on the back of the prescription, the patient’s full name, address, and his national ID/lqama or passport number.
High-Alert medications
High-Alert medications are drugs that have a heightened risk of causing significant patient harm when they are used in error. Although mistakes may or may not be more common with these drugs, the consequences of an error are clearly more devastating to patients. High Alert meds have a higher risk of causing injectionury, either as a result of a narrow therapeutic range or due to a high incidence of reported serious errors. Methods to reduce error include strategies such as improving access to information about these drugs; limiting access to High-Alert medications; using Tallman lettering, using auxiliary labels and automated alerts; standardizing the ordering, storage, preparation, and administration of these products; and employing redundancies such as automated or independent double checks when necessary. New formulary medications and additional relevant safety information will be reviewed for inclusion on the High-Alert Medication list by the Pharmacy and Therapeutics committee.
The MOH Pharmacy and Therapeutics Committee, with the input of the Medication Safety Committee, has reviewed the formulary and trend analysis of medication errors to determine a list of High-Risk/High Alert medications. The primary objective is to provide the highest quality pharmaceutical care with the minimum number of medication errors and the lowest potential for patient risk. The primary goal is to promote the fact that medication safety should be a critical component in any hospital’s overall strategic plan. The tool also provides guidance for how to incorporate medication safety into the strategic planning process.


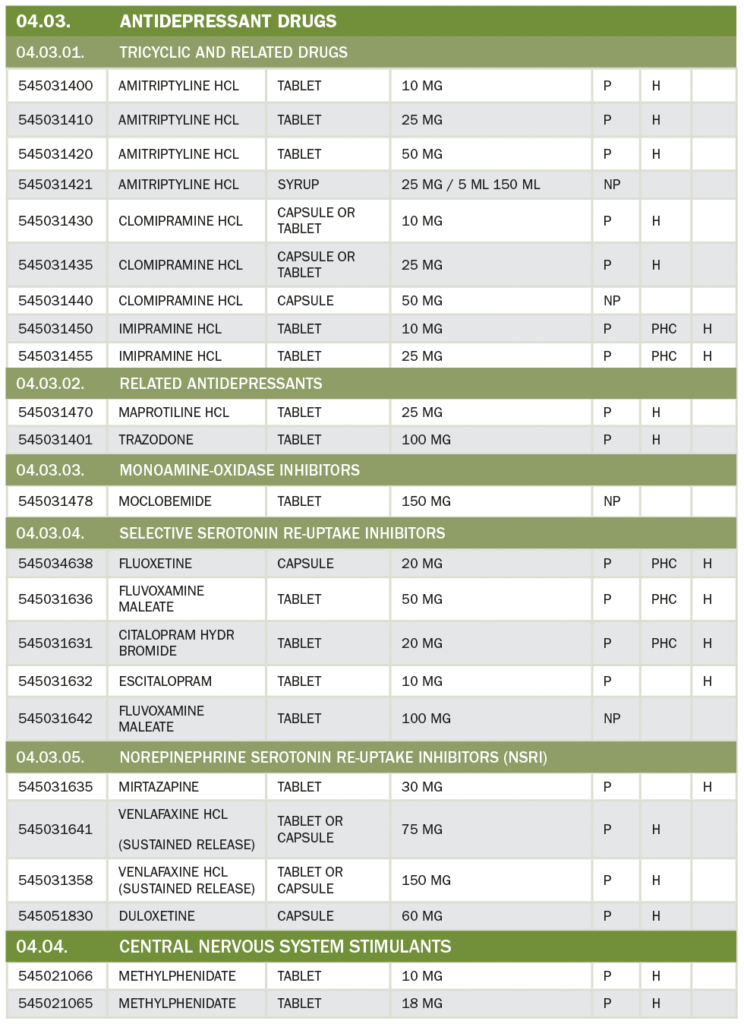
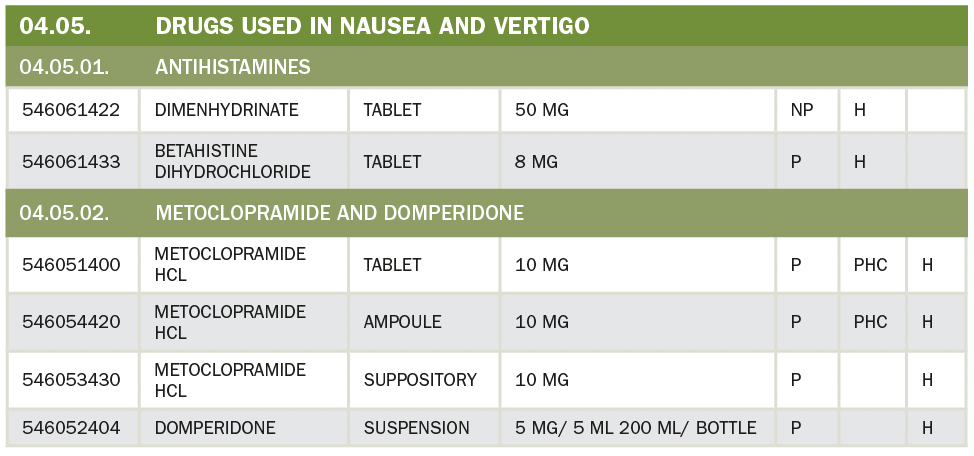
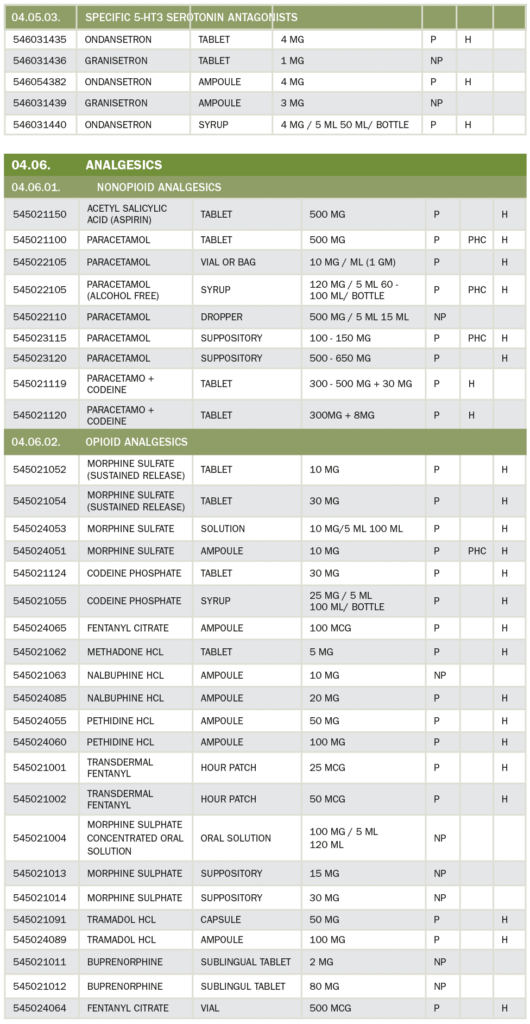
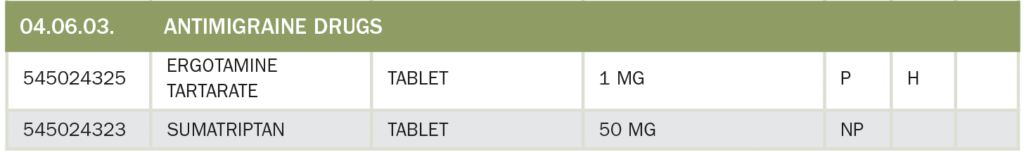
29. Who can prescribe Opioid Drugs?
Authorized practitioners may prescribe ‘opioids’ under the ambit of Saudi Law.
30. Is there a list of doctors authorized to prescribe Opioid Drugs?
Yes, there is a list of licensed doctors permitted to authorize the prescription of such drugs.
31. What approvals or notifications are required to prescribe Opioid Drugs?
The approval process has been elucidated above.
32. Which organizations are authorized to sell/distribute Opioid Drugs available?
Only government entities and government licensed entities may distribute such drugs.
33. Is there a list of retailers/distributors authorized to sell Opioid Drugs?
There isn’t an extensive list of distributors, especially in the retail space, as such medicines/ drugs are highly controlled.
34. Are there proposals for reform or significant change to the regulation of Opioid Drugs?
Yes, there are proposals to bring about change to the space on controlled medicines, however, the timeline or executionary means to its implementation are yet to be known.
35. When are they likely to come into force?
Currently unknown, indefinite.



